|
|
|
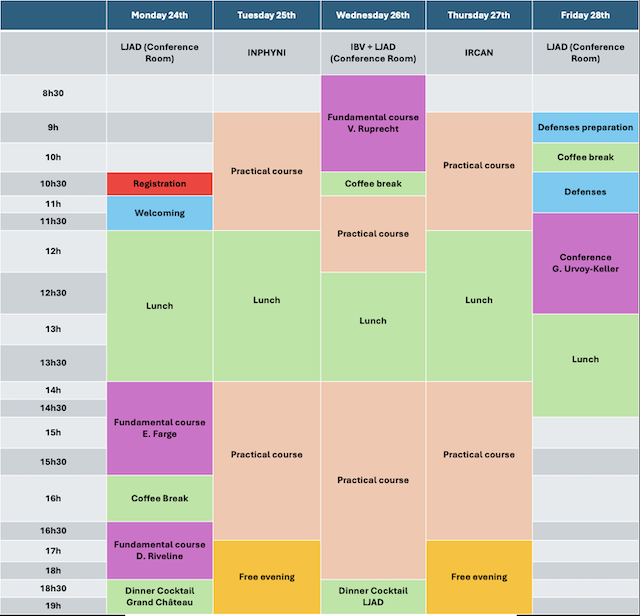
ProgramThe school will include both fundamental and practical courses. The fundamental courses will take place at the Laboratoire Jean Alexandre Dieudonné, whereas the practical courses will be performed at the IRCAN, INPHYNI and IBV.
Fundamental courses
From mechanotransduction behavioural evolutionary origins of first metazoans to tumorigenic mechanical induction The evolutionary emergence of the primitive gut in metazoans, one of the decisive events that conditioned the major evolutionary transition leading to the origin of animals, is thought to have been intimately associated with the formation of multicellular invagination (i.e. gastrulation) and its differentiation as an endomesoderm. However, the biochemical signals at the origin of gastrulation and its endomesoderm specification remain uncertain. Indeed, we will see that the different biochemical pathways involved in modern animals early embryos endomesoderm formation are not widely conserved across suerphyla. Interestingly, activation of Myo-II, and of the b-cat pathway by phosphorylation of Y654-bcat, has been shown to be triggered by mechanotransduction to lead to mesoderm invagination in Drosophila embryos and endomesoderm specification during gastrulation and epiboly in Drosophila and We will describe how hydrodynamic mechanical stresses, reminiscent of soft marine flow, trigger gastrulation and tissue inversion via a myosin-dependent mechanotransductive process in the metazoan Nematostella vectensis (Cnidaria) and the multicellular choanoflagellate Choanoeca flexa, considered to be the closest living relative of the metazoans. We also describe that, as in bilaterian animals, gastrulation in the cnidarian Nematostella vectensis induces biochemical specification of the endomesoderm through mechanical activation of the b-catenin pathway via phosphorylation of Y654. We will see that these observations suggest that the primitive emergence of the intestine in Metazoa may have been initiated by marine mechanical constraints in multicellular pre-Metazoa more than 700 million years ago, thanks to the mechanosensitive properties of Myosin, which were crucial for this evolutionary transition. A process carried out by the specification of the endomesoderm via mechanosensitive proteins containing Y654-b-catenin enabled evolutionary emergence in the first Metazoa and is specifically conserved in all Metazoa3,4. As part of a putatively inherited reminiscent process, it was found that spontaneous myogenic gastric pulses mechanotransductively inducing physiological levels of b-catenin-dependent stem cells in the colon of now-adult mice5. With pathological amplification to tumorigenic levels in neighbouring healthy cells compressed by tumour growth pressure, in vivo by using magnetic5,6 and ultrasonic tools. 1 Pouille, P. A., et al. Science signaling 2, ra16 (2009). [pii] 10.1126/scisignal.20000982 Brunet, T. et al. Nature communications 4, 2821 (2013).3 Nguyen, N. M. et al. bioRxiv 2020.12.03.407668 (2020).4 Nguyen, N. M. et al. Front Cell Dev Biol 10, 992371 (2022).5 Nguyen Ho-Bouldoires, T. H. et al. Commun Biol 5, 137 (2022).6 Fernandez-Sanchez, M. E. et al. Nature 523, 92-95 (2015).
Symmetry breaking and collective effects in biological physics Biological cells move, divide, change their shapes, adhere to their neighbors and environments to form tissues and organs. These phenomena are essential for a wide variety of biological processes during morphogenesis for example but their mesoscopic origins are often yet not clarified. To characterize them, out-of-equilibrium dynamics can be studied with physical experimental designs and associated theories. These topics have triggered new physical formalisms which call for original experimental calibrations and tests associating tightly quantitative biology with the design of new setups and models for living matter. I will illustrate these experiments of biological physics with the following examples : spontaneous breaking of symmetry for single cell motion, collective effects in elongation of epithelial colonies, spontaneous rotations in 2D and in 3D. These phenomena will show that basic principles in physics can be used and challenged to unravel new cellular mechanisms with physiological relevance.
Mechanobiology and error correction in the early embryo Mechanical forces are key for building the form and functional architecture of an organism. Development is also remarkably robust despite noise and errors at the single cell level. In this lecture I will discuss how tissue architecture and cell shape influence mechanical cell properties and cellular dynamics and how mechano-signalling pathways ensure cell plasticity and error correction during development. I will present quantitative in vitro and in vivo methods that we employ in our lab to investigate these questions and discuss recent findings how actin cytoskeletal dynamics regulate cell clearance to ensure robust embryo development. Practical courses
This practical course delves into the application of Atomic Force Microscopy (AFM) for analyzing skin cell and tissue mechanics. Participants will learn specialized techniques for preparing and handling skin samples , from individual keratinocytes to complex dermal-epidermal junction specimens. The course covers AFM operational modes optimized for soft biological materials (dermis), including point&shoot methods and high-resolution imaging of ultrastructure (cells). Students will gain hands-on experience in measuring mechanical properties such as elasticity and adhesion forces of biological samples at various levels of organization. Special attention will be given to correlating AFM data with skin physiology, including studies on aging, wound healing, and dermatological conditions. By the course's end, participants will be proficient in designing AFM experiments to quantitatively assess skin biomechanics, providing valuable insights into applications.
This practical course will focus on microfluidics techniques. After a theoretical overview, several simultaneous workshops for subgroups will allow to experiment : i) microfabrication techniques in clean room (soft lithography and PDMS channel fabrication) and ii) handling microfluidics devices under microscopes using pressure control, chemical gradient generation, deformation measurements and study of a natural microswimmers under confinement.
This workshop will provide the theoretical basis of light sheet microscopy with a focus on multi-view light sheet coupled with multiphoton laser manipulation. The students will have the possibility to practice embryo mounting and perform fast volumetric imaging coupled to laser dissection or optogenetics. For this workshop we will use the early developing Drosophila embryo as model system.
|